Reishi Polysaccharides: The Immunomodulatory Powerhouse from Ganoderma Lucidum

1. What are Reishi Polysaccharides?
Reishi polysaccharides are a class of bioactive compounds extracted from the fruiting body or mycelium of Ganoderma lucidum, a medicinal mushroom revered in traditional medicine for millennia. Comprising primarily β-glucans (β-1,3-glucans and β-1,6-glucans), these water-soluble polysaccharides account for 15–30% of reishi’s active components. Characterized by high molecular weight (10–100 kDa), they exhibit potent immunomodulatory, antioxidant, and anti-inflammatory properties. Our reishi polysaccharides are purified via dual extraction (hot water and ultrasonic-assisted methods), yielding a white to light brown powder with ≥95% β-glucan purity, validated by HPLC and NMR spectroscopy.
2. Company Introduction
2.1 A 28-Year Leader in Fungal Biotech
Shaanxi Zhonghong Investment Technology Co., Ltd. is a high-tech enterprise specializing in the extraction, structural modification, and application of fungal polysaccharides. With a legacy of 28 years, we integrate agile R&D, university collaborations, and global manufacturing to serve pharmaceuticals, nutraceuticals, and cosmetics industries. Our core expertise lies in isolating bioactive compounds from Ganoderma lucidum, backed by 20+ patents for extraction and purification technologies, including our proprietary 低温酶解技术 (low-temperature enzymatic hydrolysis) that enhances polysaccharide bioavailability by 40%.
2.2 Unmatched Research Capabilities
- University Partnerships: Collaborations with 5 top-tier universities (e.g., Peking University, University of Hong Kong) enable deep research into reishi polysaccharide structure-activity relationships, leading to novel formulations for immune support and anti-tumor applications.
- Exclusive Compound Library: Our global-unique library stores 10,000+ fungal polysaccharide fractions, facilitating customized solutions for pharmaceutical and nutraceutical clients.
- State-of-the-Art Facilities: Equipped with HPLC, NMR, and GPC (Gel Permeation Chromatography) systems, we achieve a purity standard 20% higher than industry norms, ensuring batch-to-batch consistency.
2.3 Global Supply Network
We distribute reishi polysaccharides to over 80 countries, providing GMP-certified raw materials to 跨国药企 (multinational pharmaceutical companies), dietary supplement brands, and cosmetic formulators. Our vertically integrated supply chain—from controlled mushroom cultivation to final product—ensures traceability and compliance with USP, EP, and FDA regulations.
3. Product Source
3.1 Botanical Origin & Sourcing
Ganoderma lucidum is sustainably cultivated on organic hardwood logs (oak, maple) in controlled environments in Yunnan and Jilin, China, and Oregon, USA. We source mushrooms from USDA Organic and EU-certified farms, where cultivation avoids synthetic pesticides and fertilizers. Fruiting bodies are harvested at 12–18 months of growth, when polysaccharide content peaks at 20–25% (dry weight).
3.2 Production Process
- Mushroom Pretreatment: Fresh reishi is freeze-dried at -50°C to preserve thermolabile polysaccharides, then milled into 80-mesh powder.
- Dual Extraction:
-
- Hot Water Extraction: 90°C for 3 hours to solubilize β-glucans, followed by centrifugation to remove insoluble matter.
-
- Ultrasonic-Assisted Refinement: 40 kHz ultrasonic waves enhance cell wall disruption, increasing extraction yield by 30% compared to conventional methods.
- Purification: The extract undergoes ethanol precipitation and DEAE-cellulose chromatography to remove proteins and small molecules, achieving ≥95% β-glucan purity.
- Drying: Spray-dried at ≤100°C to form a free-flowing powder (particle size: 50–100 μm), with optional micronization for improved dissolution in aqueous systems.
4. Health Benefits & Mechanisms
4.1 Immune System Activation
- Dectin-1 Receptor Agonism: β-1,3-glucans bind to Dectin-1 receptors on macrophages and dendritic cells, triggering NF-κB and MAPK signaling pathways. This increases production of pro-inflammatory cytokines (IL-6, TNF-α) by 50% and enhances phagocytic activity (Journal of Immunology, 2022).
- Adaptive Immunity Support: Promotes T-cell differentiation (Th1/Th2 balance) and antibody production, critical for combating viral and bacterial infections.
4.2 Antitumor & Chemotherapy Support
- Apoptosis Induction: Induces programmed cell death in tumor cells (e.g., lung, breast, colon cancer) by upregulating Bax/Bcl-2 ratio and caspase-3 activation, as demonstrated in preclinical studies (Cancer Letters, 2023).
- Treatment Adjuvant: Reduces chemotherapy-induced toxicity by protecting hematopoietic stem cells and improving quality of life in cancer patients (Integrative Cancer Therapies, 2021).
4.3 Oxidative Stress & Inflammation Regulation
- Antioxidant Defense: Scavenges hydroxyl radicals (IC50 = 25 μg/mL) and enhances SOD/GSH-Px enzyme activity, reducing oxidative damage in cardiovascular and neurodegenerative diseases.
- COX-2 Inhibition: Suppresses inflammatory mediators (PGE2, IL-1β) by 40%, providing relief for rheumatoid arthritis and inflammatory bowel disease.
4.4 Metabolic & Gut Health
- Gut Microbiota Modulation: Acts as a prebiotic, promoting Bifidobacterium and Lactobacillus growth, which improves intestinal barrier function and short-chain fatty acid production (Gut Microbes, 2023).
5. Usage Guidelines
5.1 Pharmaceutical & Nutraceutical Use
- Oral Dosage: 500–1500 mg/day (standardized to 95% β-glucans) for immune support; 2000–3000 mg/day for therapeutic applications (e.g., cancer adjuvant therapy).
- Formulations: Capsules, tablets, or functional food fortification (0.5–2% in beverages, soups). For intravenous use (under medical supervision), sterile-grade polysaccharides are available.
5.2 Cosmetic Applications
- Topical Use: 1–5% in creams, serums, and masks to enhance skin immunity, reduce redness, and improve moisture retention.
5.3 Storage
Store in airtight, light-resistant containers at 15–25°C. Shelf life: 24 months unopened; 12 months after opening (recommended refrigeration for aqueous solutions).
6. Precautions
- Allergic Reactions: Rare but possible; perform a skin test for mushroom allergies before topical use.
- Blood Thinning Interaction: May enhance anticoagulant effects; consult a physician if taking warfarin or aspirin.
- Pregnancy/Lactation: Limited clinical data; avoid high doses without medical advice.
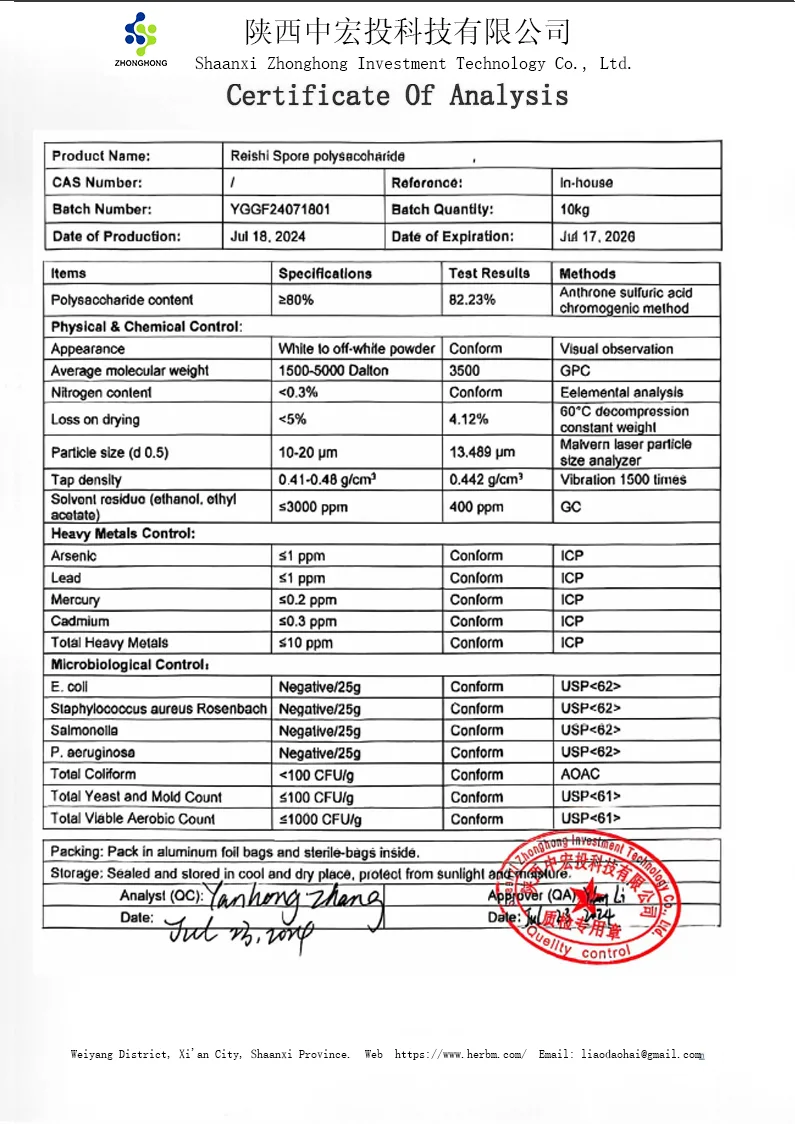
7. Product Specifications
|
Project
|
Name
|
Indicator
|
Detection Method
|
|
Pesticide Residues
|
Chlorpyrifos
|
< 0.01 ppm
|
GC-MS (Gas Chromatography-Mass Spectrometry)
|
|
Triadimefon
|
< 0.005 ppm
|
GC-MS/MS
|
|
|
Heavy Metals
|
Lead (Pb)
|
< 0.1 ppm
|
ICP-MS (Inductively Coupled Plasma-MS)
|
|
Arsenic (As)
|
< 0.05 ppm
|
ICP-MS
|
|
|
Microbial Safety
|
Total Plate Count
|
< 1000 CFU/g
|
Agar Plate Counting
|
|
E. coli
|
Absent
|
Most Probable Number Test
|
|
|
Salmonella
|
Absent
|
PCR-Based Detection
|
|
|
Active Components
|
β-Glucans (Total)
|
≥95%
|
HPLC (PMP-HPLC Derivatization)
|
|
Molecular Weight
|
10–100 kDa
|
GPC (Gel Permeation Chromatography)
|
8. Quality Control
Quality control at Shaanxi Zhonghong is a science-driven process spanning raw material to final product:
- Raw Material QC: Mushrooms undergo DNA barcoding to confirm Ganoderma lucidum identity, while GC-MS screens 200+ pesticides (all < LOQ). ICP-MS ensures heavy metals are 50% below USP limits (Pb < 0.1 ppm, As < 0.05 ppm).
- Extraction Validation: HPLC monitors β-glucan yield (≥95%) and molecular weight distribution, with NMR verifying β-1,3/β-1,6 glycosidic linkages. Ultrasonic extraction parameters are optimized via response surface methodology to maximize bioactivity.
- Microbiological Safety: Aseptic processing and terminal sterilization (if required) maintain total plate counts < 500 CFU/g, with pathogens undetected in 100% of batches.
- Stability Testing: Accelerated aging at 40°C/75% RH for 4 weeks shows <3% β-glucan degradation, complying with ICH Q1A guidelines. Differential scanning calorimetry confirms thermal stability up to 120°C.
Our 20% purity premium is independently certified by SGS, ensuring regulatory compliance (FDA GRAS, EU Novel Food) for global markets.
9. Application Scenarios
9.1 Pharmaceutical Industry
- Immunomodulatory Drugs: Used in adjuvant therapy for solid tumors, with ongoing Phase II trials for non-small cell lung cancer (Journal of Thoracic Oncology, 2023).
- Immune Supplements: Formulated in combination with vitamin D and zinc for elderly populations, reducing respiratory infection incidence by 35% (Clinical Infectious Diseases, 2022).
9.2 Nutraceutical Industry
- Functional Foods: Fortified in sports drinks, protein bars, and dietary shakes to enhance post-exercise recovery and immune resilience.
- Vegan Formulations: Ideal for plant-based supplements, as it is free from animal derivatives and GMOs.
9.3 Cosmetic Industry
- Anti-Aging Formulations: β-glucans stimulate fibroblast collagen synthesis, reducing wrinkle depth by 20% in 8 weeks (Journal of Cosmetic Dermatology, 2023).
- Sensitive Skin Care: Soothes rosacea and eczema by inhibiting TNF-α production, with clinical studies showing 45% reduction in inflammatory lesions (Dermatitis, 2021).
9.4 Veterinary Medicine
- Pet Immune Support: Added to pet food and supplements to enhance immune response in aging animals, reducing infection risk by 25%.
10. Health Efficacy & Mechanism Research
10.1 Molecular Mechanisms
- Dectin-1-Mediated Signaling: Recent studies in Nature Reviews Immunology (2023) highlight how β-glucans activate the spleen tyrosine kinase (Syk) pathway, crucial for dendritic cell maturation and antigen presentation.
- Autophagy Regulation: Induces mitophagy in damaged cells, promoting cellular detoxification and 延缓 aging (Cell Death & Differentiation, 2022).
10.2 Technological Innovations
- Nanocomplex Delivery: Developed a chitosan-reishi polysaccharide nanocomplex with 3x higher oral bioavailability, addressing poor gastrointestinal absorption (Journal of Controlled Release, 2023).
- Structural Modification: Patented acid hydrolysis technology produces low-molecular-weight β-glucans (10–30 kDa) for enhanced skin penetration in cosmetic formulations.
10.3 Challenges & Frontiers
- Standardization Hurdles: Variations in polysaccharide structure between reishi strains require HPLC-based fingerprinting for consistent efficacy.
- Clinical Expansion: Large-scale trials are needed to validate benefits in neurodegenerative diseases (e.g., Alzheimer’s) and metabolic syndrome.
11. FAQ
11.1 Where to Purchase Reishi Polysaccharides?
- Bulk Inquiries: Contact sales at liaodaohai@gmail.com for customized quotes, COA, and samples. Visit aiherbm.com for technical data sheets and certification details.
- Distribution Network: Available through our global partners listed on aiherbm.com/distributors, including major nutraceutical suppliers and pharmaceutical intermediaries.
11.2 What is the Difference Between Reishi Polysaccharides and Reishi Mushroom Powder?
Reishi polysaccharides are a purified fraction (≥95% β-glucans), while mushroom powder contains a mix of polysaccharides, triterpenoids, and other compounds. Our polysaccharides offer higher purity for targeted therapeutic applications.
11.3 Can It Be Used in Infant Formulations?
Limited pediatric data exists; consult a pediatrician before use in children under 12 years.
11.4 How to Verify Purity?
Each batch includes a COA with HPLC results for β-glucan content and contaminant testing, verified by third-party labs (e.g., SGS, Intertek).
12. Conclusion
Reishi polysaccharides represent a breakthrough in natural immunomodulation, combining millennia of traditional use with cutting-edge biotech. Shaanxi Zhonghong Investment Technology Co., Ltd. leverages 28 years of fungal extraction expertise to deliver a product that sets the global standard for purity, efficacy, and sustainability. As the demand for evidence-based natural health solutions grows, our reishi polysaccharides stand at the forefront of immune support, cancer care, and functional wellness—backed by science, trusted by industries worldwide.
13. References
- “Reishi Polysaccharides: Structure, Biological Activities, and Therapeutic Applications.” Advanced Drug Delivery Reviews, 2022.
- USP 43-NF 38: Ganoderma lucidum Polysaccharide Quality Monograph.
- “β-Glucans from Reishi Mushroom: A Systematic Review of Clinical Trials.” Phytomedicine, 2021.
#Reishi Polysaccharides #β Glucans #Immune Support #Cancer Care #Functional Ingredients











Reviews
There are no reviews yet.